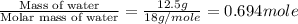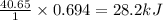Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2


Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
The enthalpy of vaporization of liquid water is 40.65 kJ/mol. Calculate the energy required to vapor...
Questions


English, 04.02.2020 00:05

Mathematics, 04.02.2020 00:05





Mathematics, 04.02.2020 00:05

Health, 04.02.2020 00:05


Mathematics, 04.02.2020 00:05

Mathematics, 04.02.2020 00:05

Computers and Technology, 04.02.2020 00:05