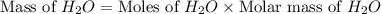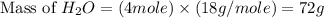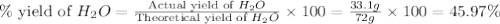Chemistry, 26.08.2019 12:50 dbenitezmontoya3
Two moles of oxygen gas is reacted with hydrogen gas to produce water in this reaction: 2 h2 + o2 → 2 h2o in the lab you actually made 33.1 grams of water. what is the percent yield? 218.10% 91.80% 45.87% 54.50%

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2


Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
Two moles of oxygen gas is reacted with hydrogen gas to produce water in this reaction: 2 h2 + o2 →...
Questions



World Languages, 08.12.2021 18:40

Mathematics, 08.12.2021 18:40

Mathematics, 08.12.2021 18:40


Mathematics, 08.12.2021 18:40


Mathematics, 08.12.2021 18:40


History, 08.12.2021 18:40

Chemistry, 08.12.2021 18:40


English, 08.12.2021 18:40



Mathematics, 08.12.2021 18:40



SAT, 08.12.2021 18:40

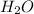 is, 45.97 %
is, 45.97 %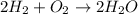
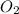 react to give 2 moles of
react to give 2 moles of 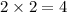 moles of
moles of