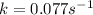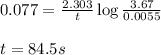Chemistry, 11.02.2020 23:54 AutumnGarringer
Suppose the half-life is 9.0 s for a first order reaction and the reactant concentration is 0.0741 M 50.7 s after the reaction starts. How many seconds after the start of the reaction does it take for the reactant concentration to decrease to 0.0055 M?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3


Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

You know the right answer?
Suppose the half-life is 9.0 s for a first order reaction and the reactant concentration is 0.0741 M...
Questions


Mathematics, 23.12.2020 03:00

Chemistry, 23.12.2020 03:00

Mathematics, 23.12.2020 03:00



Computers and Technology, 23.12.2020 03:10



Physics, 23.12.2020 03:10


Social Studies, 23.12.2020 03:10

Mathematics, 23.12.2020 03:10

Geography, 23.12.2020 03:10

Biology, 23.12.2020 03:10


Mathematics, 23.12.2020 03:10

English, 23.12.2020 03:10

Business, 23.12.2020 03:10

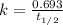
 = half-life of the reaction = 9.0 s
= half-life of the reaction = 9.0 s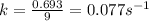
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0507/4595/f1041.png) ......(1)
......(1)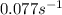
![[A_o]](/tpl/images/0507/4595/dc622.png) = initial amount of the reactant = ?
= initial amount of the reactant = ?![0.077=\frac{2.303}{50.7}\log\frac{[A_o]}{0.0741}](/tpl/images/0507/4595/3671b.png)
![[A_o]=3.67M](/tpl/images/0507/4595/2dfb2.png)
![[A]=0.0055M](/tpl/images/0507/4595/a1973.png)