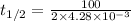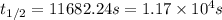Chemistry, 11.02.2020 21:27 litzyguzman13
In a zero order reaction, it takes 342 seconds for 75% of a hypothetical reaction to decompose. Determine the half-life t1/2} in units of seconds. Do not enter units with your numerical answer.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1


Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
In a zero order reaction, it takes 342 seconds for 75% of a hypothetical reaction to decompose. Dete...
Questions

Mathematics, 23.12.2019 02:31



Mathematics, 23.12.2019 02:31

Biology, 23.12.2019 02:31

History, 23.12.2019 02:31


Social Studies, 23.12.2019 02:31

World Languages, 23.12.2019 02:31



History, 23.12.2019 02:31


Chemistry, 23.12.2019 02:31

Mathematics, 23.12.2019 02:31


Physics, 23.12.2019 02:31



English, 23.12.2019 02:31


![\ln [A]=-kt+\ln [A_o]](/tpl/images/0507/1434/bdc3f.png)
![[A_o]](/tpl/images/0507/1434/dc622.png) = let initial concentration = 100
= let initial concentration = 100![[A]](/tpl/images/0507/1434/6aa06.png) = final concentration = 25
= final concentration = 25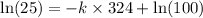
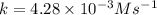
![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0507/1434/b5b11.png)