
Chemistry, 11.02.2020 21:21 Svetakotok
A particular oral contraceptive contains 0.038 mg of ethinyl estradiol in each pill. The formula of this compound is C20H24O2. How many molecules of ethinyl estradiol are there in one pill?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
You know the right answer?
A particular oral contraceptive contains 0.038 mg of ethinyl estradiol in each pill. The formula of...
Questions

English, 28.01.2022 05:10



Mathematics, 28.01.2022 05:10


Law, 28.01.2022 05:10


Mathematics, 28.01.2022 05:10

Social Studies, 28.01.2022 05:10

Mathematics, 28.01.2022 05:10

Biology, 28.01.2022 05:10

Physics, 28.01.2022 05:10




Computers and Technology, 28.01.2022 05:10





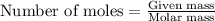
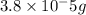 (Conversion factor: 1 g = 1000 mg)
(Conversion factor: 1 g = 1000 mg)![(C_{20}H_{24}O_2)=[(20\times 12)+(24\times 1)+(2\times 16)]=296g/mol](/tpl/images/0507/1249/cf315.png)
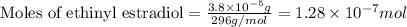
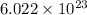 number of molecules
number of molecules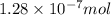 of ethinyl estradiol will contain =
of ethinyl estradiol will contain = 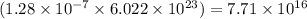 number of molecules
number of molecules

