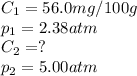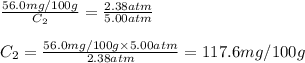Chemistry, 11.02.2020 19:23 Whitehouse9
At a certain temperature, the solubility of N₂ gas in water at 2.38 atm is 56.0 mg of N₂ gas/100 g water. Calculate the solubility of N₂ gas in water, at the same temperature, if the partial pressure of N₂ gas over the solution is increased from 2.38 atm to 5.00 atm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
At a certain temperature, the solubility of N₂ gas in water at 2.38 atm is 56.0 mg of N₂ gas/100 g w...
Questions


Mathematics, 03.02.2021 03:30

Mathematics, 03.02.2021 03:30






Mathematics, 03.02.2021 03:30

Mathematics, 03.02.2021 03:30

Computers and Technology, 03.02.2021 03:30


Mathematics, 03.02.2021 03:30

Physics, 03.02.2021 03:30


Biology, 03.02.2021 03:30

Mathematics, 03.02.2021 03:30



Chemistry, 03.02.2021 03:30

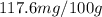
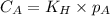
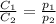
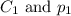 are the initial concentration and partial pressure of nitrogen gas
are the initial concentration and partial pressure of nitrogen gas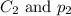 are the final concentration and partial pressure of nitrogen gas
are the final concentration and partial pressure of nitrogen gas