Chemistry, 11.02.2020 04:39 ivethzurita0425
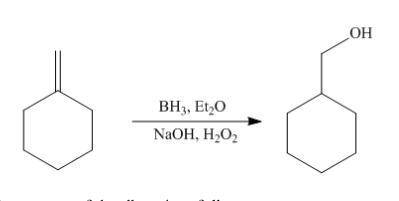
Hydroboration-oxidation of an alkene, C7H12, gives a single product that exhibits 13C spectral data below.
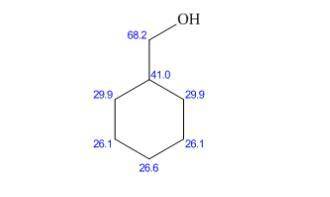
Broadband-decoupled 13C NMR: 26.1, 26.9, 29.9, 40.5, 68.2 δ
DEPT-90: 40.5 δ
DEPT-135: positive peaks at 40.5; negative peaks at 26.1, 26.9, 29.9, 68.2 δ
Draw a structure for this compound.
You do not have to consider stereochemistry.
You do not have to explicitly draw H atoms.
In cases where there is more than one answer, just draw one.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1
You know the right answer?
Hydroboration-oxidation of an alkene, C7H12, gives a single product that exhibits 13C spectral data...
Questions

History, 21.07.2019 15:30


Mathematics, 21.07.2019 15:30



Computers and Technology, 21.07.2019 15:30





Mathematics, 21.07.2019 15:30



Mathematics, 21.07.2019 15:30

Mathematics, 21.07.2019 15:30



Computers and Technology, 21.07.2019 15:30


Computers and Technology, 21.07.2019 15:30

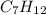
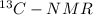 spectrum of alcohol formed by the Hydroboration - oxidation reaction of an alkene we can say
spectrum of alcohol formed by the Hydroboration - oxidation reaction of an alkene we can say 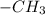 and negative peaks that corresponds to -
and negative peaks that corresponds to -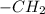 .It shows positive peaks at 40.5δ that indicates the -CH group and the remaining negative peaks at 26.1 δ,26.9δ.29.9δ δ and 68.2δ.This indicates that the compound has 4 non-equivalent
.It shows positive peaks at 40.5δ that indicates the -CH group and the remaining negative peaks at 26.1 δ,26.9δ.29.9δ δ and 68.2δ.This indicates that the compound has 4 non-equivalent