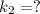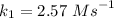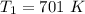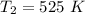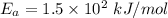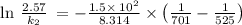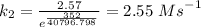Chemistry, 11.02.2020 04:04 ashhull2002
The reaction between nitrogen dioxide and carbon monoxide is NO2(g)+CO(g)→NO(g)+CO2(g)NO2(g)+CO( g)→NO(g)+CO2(g) The rate constant at 701 KK is measured as 2.57 M−1⋅s−1M−1⋅s−1 and that at 895 KK is measured as 567 M−1⋅s−1M−1⋅s−1. The activation energy is 1.5×102 1.5×102 kJ/molkJ/mol. Predict the rate constant at 525 KK . Express your answer in liters per mole-second to three significant figures.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
The reaction between nitrogen dioxide and carbon monoxide is NO2(g)+CO(g)→NO(g)+CO2(g)NO2(g)+CO( g)→...
Questions

Mathematics, 13.04.2020 08:03

Computers and Technology, 13.04.2020 08:03

Mathematics, 13.04.2020 08:03


Mathematics, 13.04.2020 08:03


Mathematics, 13.04.2020 08:04

Mathematics, 13.04.2020 08:04

Chemistry, 13.04.2020 08:04



Chemistry, 13.04.2020 08:04


Chemistry, 13.04.2020 08:05






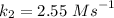
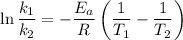
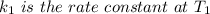
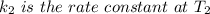
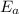 is the activation energy
is the activation energy