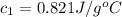Chemistry, 11.02.2020 01:14 treseanthegreat
A piece of unknown metal with mass 30 g is heated to 110.0 °C and dropped into 100.0 g of water at 20.0 °C. The final temperature of the system is 25.0 °C. Determine the specific heat of the metal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
A piece of unknown metal with mass 30 g is heated to 110.0 °C and dropped into 100.0 g of water at 2...
Questions

Mathematics, 27.01.2021 19:10

English, 27.01.2021 19:10

Mathematics, 27.01.2021 19:10

Health, 27.01.2021 19:10

Chemistry, 27.01.2021 19:10

Mathematics, 27.01.2021 19:10




Mathematics, 27.01.2021 19:10


English, 27.01.2021 19:10

History, 27.01.2021 19:10



History, 27.01.2021 19:10



Health, 27.01.2021 19:10

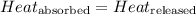
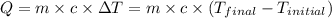
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0505/6952/09236.png) ......(1)
......(1)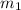 = mass of metal = 30 g
= mass of metal = 30 g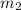 = mass of water = 100 g
= mass of water = 100 g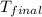 = final temperature = 25°C
= final temperature = 25°C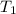 = initial temperature of metal = 110°C
= initial temperature of metal = 110°C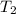 = initial temperature of water = 20.0°C
= initial temperature of water = 20.0°C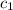 = specific heat of metal = ?
= specific heat of metal = ?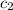 = specific heat of water = 4.186 J/g°C
= specific heat of water = 4.186 J/g°C![30\times c_1\times (25-110)=-[100\times 4.186\times (25-20)]](/tpl/images/0505/6952/ee389.png)