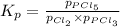Chemistry, 11.02.2020 00:56 cia196785920
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas. pcl3(g) + cl2(g) equilibrium reaction arrow pcl5(g) a gas vessel is charged with a mixture of pcl3(g) and cl2(g), which is allowed to equilibriate at 450 k. at equilibrium the partial pressures of the three gases are ppcl3 = 0.124 atm, pcl2 = 0.157 atm, and ppcl5 = 1.30 atm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas. pcl3(g) + cl...
Questions





Biology, 31.07.2019 03:30


Biology, 31.07.2019 03:30




Social Studies, 31.07.2019 03:30

History, 31.07.2019 03:30

Biology, 31.07.2019 03:30

Physics, 31.07.2019 03:30






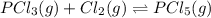
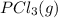 and
and 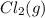 , which is allowed to equilibriate at 450 K. At equilibrium the partial pressures of the three gases are
, which is allowed to equilibriate at 450 K. At equilibrium the partial pressures of the three gases are 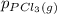 = 0.126 atm ,
= 0.126 atm , 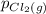 = 0.156 atm , and
= 0.156 atm , and 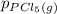 = 1.60 atm. What is the value of
= 1.60 atm. What is the value of  at this temperature?
at this temperature?