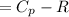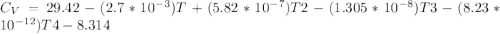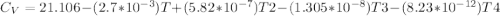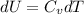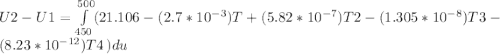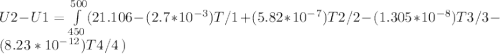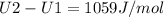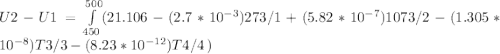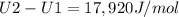The ideal gas heat capacity of nitrogen varies with temperature. It is given by:
Cp = 29.42 -...

Chemistry, 10.02.2020 23:04 steventhecool22
The ideal gas heat capacity of nitrogen varies with temperature. It is given by:
Cp = 29.42 - (2.170*10^-3 ) T + (0.0582*10^-5 ) T2 + (1.305*10^-8 ) T3 – (0.823*10^-11) T4
T in K and Cp in Joule/(mole-K). Assuming that N2 is an ideal gas:
A) How much internal energy (per mole) must be added to nitrogen to increase its temperature from 450 to 500 K. B) Repeat part A for an initial temperature of 273 K and final temperature of 1073 K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Questions

Mathematics, 27.04.2020 01:21


Mathematics, 27.04.2020 01:21

Mathematics, 27.04.2020 01:21

Biology, 27.04.2020 01:21


Mathematics, 27.04.2020 01:21

Physics, 27.04.2020 01:21

Chemistry, 27.04.2020 01:21



Social Studies, 27.04.2020 01:21

Mathematics, 27.04.2020 01:21

Mathematics, 27.04.2020 01:21



Mathematics, 27.04.2020 01:21

Biology, 27.04.2020 01:21

Geography, 27.04.2020 01:21

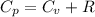
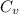 from above if we make
from above if we make