
Chemistry, 10.02.2020 23:08 eichlingkera13
Conversion of 0.702 g of liquid water to steam at 100.0°C requires 1.56 kJ of heat. Calculate the molar enthalpy of evaporation of water at 100.0°C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1
You know the right answer?
Conversion of 0.702 g of liquid water to steam at 100.0°C requires 1.56 kJ of heat. Calculate the mo...
Questions


History, 31.03.2021 07:50

Mathematics, 31.03.2021 07:50

Arts, 31.03.2021 07:50



Mathematics, 31.03.2021 07:50


English, 31.03.2021 07:50




History, 31.03.2021 07:50

English, 31.03.2021 07:50


English, 31.03.2021 07:50

English, 31.03.2021 07:50


Mathematics, 31.03.2021 07:50

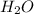 as:-
as:-
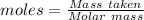
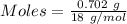
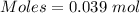
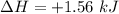
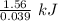 of energy is used
of energy is used

