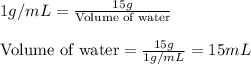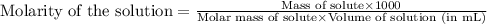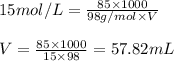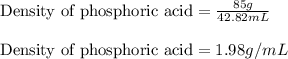Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
Concentrated phosphoric acid is sold as a solution of 85% phosphoric acid and 15% water by mass. Giv...
Questions


English, 29.01.2020 14:44

Mathematics, 29.01.2020 14:44


Mathematics, 29.01.2020 14:45


History, 29.01.2020 14:45

Biology, 29.01.2020 14:45

Mathematics, 29.01.2020 14:45

Physics, 29.01.2020 14:45

Chemistry, 29.01.2020 14:45

Mathematics, 29.01.2020 14:45


History, 29.01.2020 14:45



Mathematics, 29.01.2020 14:45


Mathematics, 29.01.2020 14:45

Biology, 29.01.2020 14:45

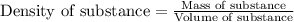 ......(1)
......(1)