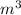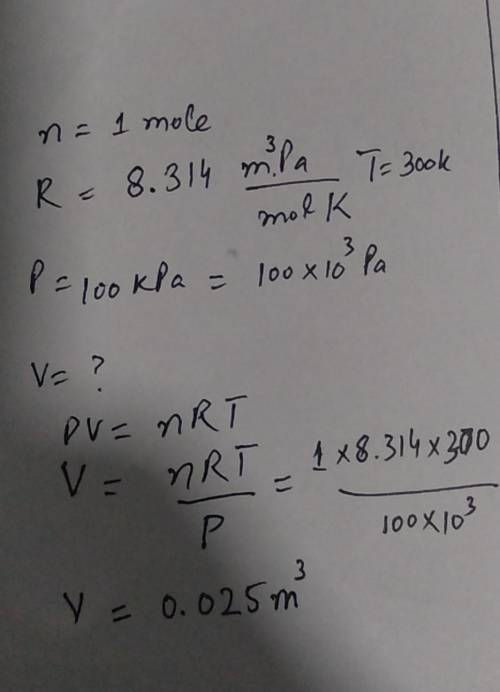A balloon is filled with 1 mole of helium gas at 100 kPa of pressure and a
temperature of 300 K...

Chemistry, 10.02.2020 21:07 cordobamariana07
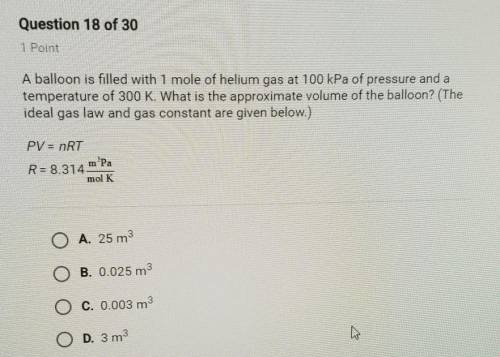
A balloon is filled with 1 mole of helium gas at 100 kPa of pressure and a
temperature of 300 K. What is the approximate volume of the balloon? (The
ideal gas law and gas constant are given below.)
PV = nRT
R=8.314 m. Pa/
mol K

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2


You know the right answer?
Questions

Mathematics, 27.08.2019 06:00

Biology, 27.08.2019 06:00





Mathematics, 27.08.2019 06:00


Biology, 27.08.2019 06:00


Mathematics, 27.08.2019 06:00





Mathematics, 27.08.2019 06:00

Biology, 27.08.2019 06:00

Mathematics, 27.08.2019 06:00

Physics, 27.08.2019 06:00