Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
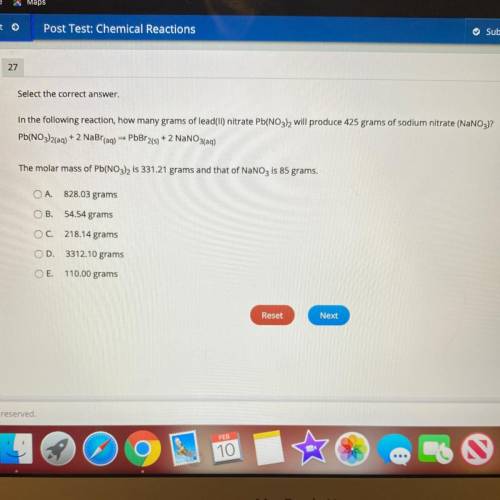
In the following reaction, how many grams of lead(II) nitrate Pb(NO3), will produce 425 grams of sod...
Questions


Mathematics, 03.02.2021 17:50

Chemistry, 03.02.2021 17:50

Mathematics, 03.02.2021 17:50

English, 03.02.2021 17:50


English, 03.02.2021 17:50

Mathematics, 03.02.2021 17:50



Mathematics, 03.02.2021 17:50




Mathematics, 03.02.2021 17:50

Arts, 03.02.2021 17:50


Computers and Technology, 03.02.2021 17:50

Mathematics, 03.02.2021 17:50

Mathematics, 03.02.2021 17:50