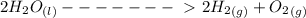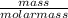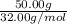Passing an electric current through a sample of water (h2o) can cause the water to decompose into hydrogen gas (h2) and oxygen gas (o2) according to the following equation.
2h2o mc026-1.jpg 2h2 + o2
the molar mass of h2o is 18.01 g/mol. the molar mass of o2 is 32.00 g/mol. what mass of h2o, in grams, must react to produce 50.00 g of o2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
You know the right answer?
Passing an electric current through a sample of water (h2o) can cause the water to decompose into hy...
Questions


Biology, 11.10.2020 14:01


World Languages, 11.10.2020 14:01


Social Studies, 11.10.2020 14:01

History, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01



Business, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01






Arts, 11.10.2020 14:01