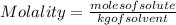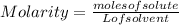The equation used to calculate the change in freezing point (δtf) of a substance is:
δtf = k...

The equation used to calculate the change in freezing point (δtf) of a substance is:
δtf = kfm
where kf is the freezing point depression constant and m is the molality of the solution. which of the statements explains why molality is used instead of molarity in this equation?
a. molality does not appear in many equations, so it is used here to distinguish this equation from other similar ones.
b. as the temperature of a solution changes, its volume will also change, which will affect its molarity but not its molality.
c. in solutions, moles are not directly related to grams and the freezing point of a solution is dependent solely on the number of grams of solute.
d. the equation was originally published with m as a typo, rather than m, but the values are close enough that the equation is still valid.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1


Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
Questions


Mathematics, 16.12.2020 19:00


Mathematics, 16.12.2020 19:00




Mathematics, 16.12.2020 19:00



Mathematics, 16.12.2020 19:00





Mathematics, 16.12.2020 19:00

Biology, 16.12.2020 19:00


History, 16.12.2020 19:00