Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
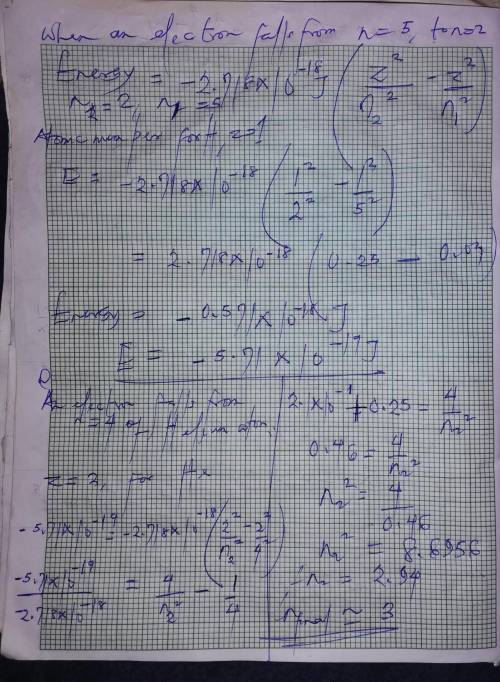
When the excited electron in a hydrogen atom falls from to , a photon of blue light is emitted. if a...
Questions


Mathematics, 06.05.2021 01:40


English, 06.05.2021 01:40

Mathematics, 06.05.2021 01:40



Mathematics, 06.05.2021 01:40


Mathematics, 06.05.2021 01:40



Mathematics, 06.05.2021 01:40


Mathematics, 06.05.2021 01:40



Mathematics, 06.05.2021 01:40

Mathematics, 06.05.2021 01:40

English, 06.05.2021 01:40