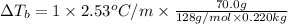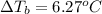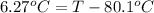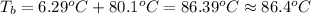Calculate the boiling point of a solution prepared by dissolving 70.0 g of naphthalene, c10h8 (a nonvolatile nonelectrolyte), in 220.0 g of benzene, c6h6. the kb for benzene = 2.53oc/m. the boiling point of pure benzene is 80.1oc.. ans: 86.4 degrees celsius. i did this so far. 1)70g c10h8(1mol c10h8/128gc10h8)= .546mol c10h8. benzene)=2.482m. 3) (2.482)(2.53 c/m)=6.289. i'm not sure if i am starting this off right, can anyone me get the correct answer? ans ty!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2


Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 09:00
Agust of wind blowing east pushes against a ball. when will the wind do work on the ball? when the ball moves to the east when the ball moves to the north when the ball stays in one place when the ball moves north or south
Answers: 1
You know the right answer?
Calculate the boiling point of a solution prepared by dissolving 70.0 g of naphthalene, c10h8 (a non...
Questions



Mathematics, 21.10.2020 22:01



English, 21.10.2020 22:01


Mathematics, 21.10.2020 22:01



History, 21.10.2020 22:01

Biology, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01

History, 21.10.2020 22:01

English, 21.10.2020 22:01



Chemistry, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01

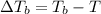
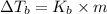
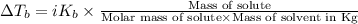
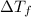 =Elevation in boiling point
=Elevation in boiling point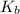 = Boiling point constant of solvent = 2.53 °C/m(benzene)
= Boiling point constant of solvent = 2.53 °C/m(benzene)