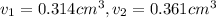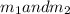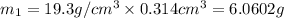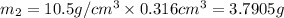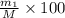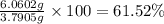Chemistry, 11.11.2019 06:31 kimloveswim
Gold is alloyed(mixed) with other metals to increase its hardness in making jewelry. (a) consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3. the jewelry contains only gold and silver, which have densities of 19.3 g/cm3 and 10.5 g/cm3, repectively. if the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains. calculate the percentage of gold(by mass) in the jewelry. (b) the relative amount of gold in an alloy is commonly expressed in units of karats.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a concentration of 5.2 × 10–8 m h3o+?
Answers: 1


Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
Gold is alloyed(mixed) with other metals to increase its hardness in making jewelry. (a) consider a...
Questions


Mathematics, 11.03.2021 08:10

Social Studies, 11.03.2021 08:10

Mathematics, 11.03.2021 08:10


Mathematics, 11.03.2021 08:10


Mathematics, 11.03.2021 08:10


Mathematics, 11.03.2021 08:10

Mathematics, 11.03.2021 08:10

Mathematics, 11.03.2021 08:10


Mathematics, 11.03.2021 08:10

Mathematics, 11.03.2021 08:10


Mathematics, 11.03.2021 08:10

Mathematics, 11.03.2021 08:10


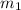
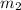
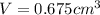
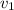
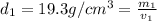
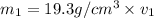
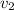
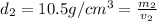
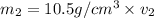
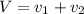
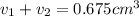 ..(1)
..(1)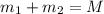
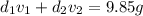
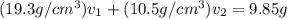 ..(2)
..(2)