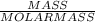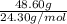Chemistry, 20.10.2019 13:00 cybilmariejensen
The molar mass of magnesium (mg) is 24.30 g/mol. there are 6.02 x 10^23 atoms in 1 mol. how many atoms are present in 48.60 g of mg?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3


Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1
You know the right answer?
The molar mass of magnesium (mg) is 24.30 g/mol. there are 6.02 x 10^23 atoms in 1 mol. how many ato...
Questions

English, 06.05.2020 02:59

Mathematics, 06.05.2020 02:59






Chemistry, 06.05.2020 02:59




Computers and Technology, 06.05.2020 02:59


English, 06.05.2020 02:59

Mathematics, 06.05.2020 02:59

Social Studies, 06.05.2020 02:59

Biology, 06.05.2020 02:59

Biology, 06.05.2020 02:59

Mathematics, 06.05.2020 02:59