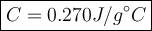2. a 237 g piece of molybdenum, initially at 100.0 °c, was dropped into 244 g of
water at 10.0...

Chemistry, 29.01.2020 14:40 alonnachambon
2. a 237 g piece of molybdenum, initially at 100.0 °c, was dropped into 244 g of
water at 10.0°c. when the system came to thermal equilibrium, the temperature
was 15.3°c. what is the specific heat capacity of molybdenum? the specific heat
capacity of water is 4.184 j/g. k. (2.5 points)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1


You know the right answer?
Questions


History, 03.02.2020 19:01

History, 03.02.2020 19:01

Computers and Technology, 03.02.2020 19:01



Mathematics, 03.02.2020 19:01


Mathematics, 03.02.2020 19:01

Mathematics, 03.02.2020 19:01

Mathematics, 03.02.2020 19:01

Geography, 03.02.2020 19:01



Mathematics, 03.02.2020 19:01

Mathematics, 03.02.2020 19:01


Mathematics, 03.02.2020 19:01