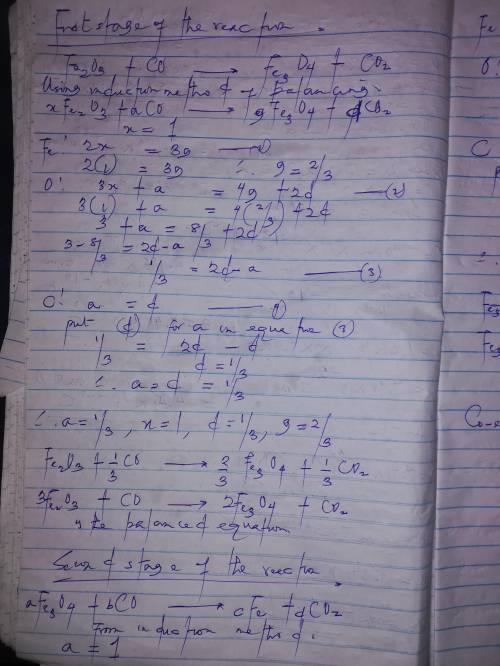In one stage in the commercial production of iron metal in a blast furnace, the iron(iii) oxide (fe2o3) reacts with carbon monoxide to form solid fe3o4 and carbon dioxide gas. in a second stage, the fe3o4 reacts further with carbon monoxide to produce solid elemental iron and carbon dioxide. what is the coefficient of iron(iii) oxide in the second stage of the balanced equations?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
In one stage in the commercial production of iron metal in a blast furnace, the iron(iii) oxide (fe2...
Questions




Mathematics, 06.05.2020 08:34

English, 06.05.2020 08:34

Mathematics, 06.05.2020 08:34

English, 06.05.2020 08:34

Mathematics, 06.05.2020 08:34

Biology, 06.05.2020 08:34

Mathematics, 06.05.2020 08:34

Mathematics, 06.05.2020 08:34

Computers and Technology, 06.05.2020 08:34

Mathematics, 06.05.2020 08:34

History, 06.05.2020 08:34



Chemistry, 06.05.2020 08:34

Mathematics, 06.05.2020 08:34

English, 06.05.2020 08:34