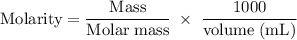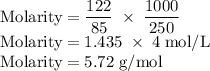Achemist prepares a solution of sodium nitrate (nano3) by measuring out 122.g of sodium nitrate into 250.ml volumetric flask and filling the flask to the mark with water. calculate the concentration in mol/l of the chemist's sodium nitrate solution. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:02
In general, as the temperature of a solvent increases, the solubility of any gas dissolved in that solvent:
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
Achemist prepares a solution of sodium nitrate (nano3) by measuring out 122.g of sodium nitrate into...
Questions

English, 09.04.2020 02:02






Spanish, 09.04.2020 02:02

Biology, 09.04.2020 02:02


Spanish, 09.04.2020 02:02

Mathematics, 09.04.2020 02:02



Chemistry, 09.04.2020 02:02


Mathematics, 09.04.2020 02:02



Social Studies, 09.04.2020 02:02