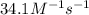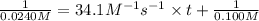Chemistry, 29.01.2020 03:52 njohns5327home
At a certain temperature the rate of this reaction is second order in nh4oh with a rate constant of 34.1 m^-1*s^-1:
nh4oh(aq) > nh3(aq) + h2o (aq)
suppose a vessel contains nh4oh at a concentration of 0.100m. calculate how long it takes for the concentration of nh4oh to decrease to 0.0240 m. you may assume no other reaction is important. round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
You know the right answer?
At a certain temperature the rate of this reaction is second order in nh4oh with a rate constant of...
Questions


Health, 02.12.2020 04:00

Mathematics, 02.12.2020 04:00

Mathematics, 02.12.2020 04:00

Mathematics, 02.12.2020 04:00


English, 02.12.2020 04:00



Business, 02.12.2020 04:00

Mathematics, 02.12.2020 04:00

Mathematics, 02.12.2020 04:00

Mathematics, 02.12.2020 04:00


Mathematics, 02.12.2020 04:00

Computers and Technology, 02.12.2020 04:00



Physics, 02.12.2020 04:00

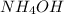 to decrease to 0.0240 M.
to decrease to 0.0240 M.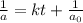
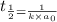
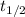 = half life of reaction
= half life of reaction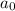 = initial concentration
= initial concentration 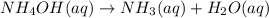
![[NH_4OH]=a_o=0.100 M](/tpl/images/0480/1114/fa6e4.png)
![[NH_4OH]=a=0.0240 M](/tpl/images/0480/1114/c0e10.png)