
Chemistry, 29.01.2020 02:45 gizmo50245
Asample of 1.000 g of a compound containing carbon and hydrogen reacts with oxygen at elevated temperature to yield 0.692 g h₂o and 3.381 g co₂. (a) calculate the masses of c and h in the sample. (b) does the compound contain any other elements

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
Asample of 1.000 g of a compound containing carbon and hydrogen reacts with oxygen at elevated tempe...
Questions

Mathematics, 31.08.2021 01:10

English, 31.08.2021 01:10



Mathematics, 31.08.2021 01:10


History, 31.08.2021 01:20



Mathematics, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20

Physics, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20


Mathematics, 31.08.2021 01:20


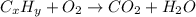
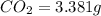
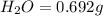
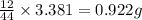 of carbon will be contained.
of carbon will be contained.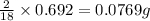 of hydrogen will be contained.
of hydrogen will be contained.

