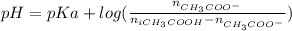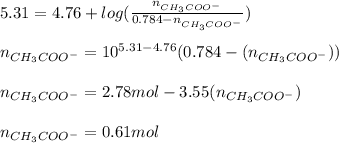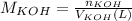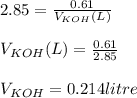Chemistry, 29.01.2020 01:45 jacksonyodell8601
You need to prepare an acetate buffer of ph 5.31 5.31 from a 0.784 m 0.784 m acetic acid solution and a 2.85 m koh 2.85 m koh solution. if you have 930 ml 930 ml of the acetic acid solution, how many milliliters of the koh koh solution do you need to add to make a buffer of ph 5.31 5.31 ? the p k a pka of acetic acid is 4.76. 4.76.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
You need to prepare an acetate buffer of ph 5.31 5.31 from a 0.784 m 0.784 m acetic acid solution an...
Questions


Advanced Placement (AP), 09.12.2020 04:00



Mathematics, 09.12.2020 04:00



Chemistry, 09.12.2020 04:00


English, 09.12.2020 04:00

Mathematics, 09.12.2020 04:00




English, 09.12.2020 04:00

Mathematics, 09.12.2020 04:00

Spanish, 09.12.2020 04:00

Chemistry, 09.12.2020 04:00

Mathematics, 09.12.2020 04:00

Mathematics, 09.12.2020 04:00

![pH=pKa + log(\frac{[CH_{3}COO^{-}]}{[CH_{3}COOH]})](/tpl/images/0479/5908/7f7e8.png)