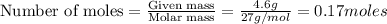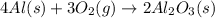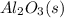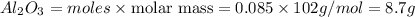Chemistry, 29.01.2020 00:42 melissareid65
Luminum and oxygen react according to the following equation: 4al(s) +3o2(g) --> 2al2o3(s) what mass of al2o3, in grams, can be made by reacting 4.6 g al with excess oxygen?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
Luminum and oxygen react according to the following equation: 4al(s) +3o2(g) --> 2al2o3(s) what...
Questions

Mathematics, 14.05.2021 18:00




Mathematics, 14.05.2021 18:00


Computers and Technology, 14.05.2021 18:00

Social Studies, 14.05.2021 18:00

Social Studies, 14.05.2021 18:00



Arts, 14.05.2021 18:00


Mathematics, 14.05.2021 18:00

Mathematics, 14.05.2021 18:00


Mathematics, 14.05.2021 18:00


Engineering, 14.05.2021 18:00

Mathematics, 14.05.2021 18:00

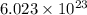 of particles.
of particles.