Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

You know the right answer?
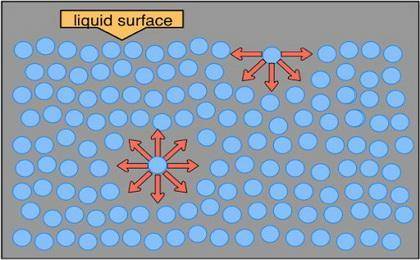
The surface tension of water is 7.28 ✕ 10−2 j/m2 at 20°c. predict whether the surface tension of hep...
Questions

Chemistry, 06.10.2019 16:30


History, 06.10.2019 16:30

Mathematics, 06.10.2019 16:30

Social Studies, 06.10.2019 16:30

Mathematics, 06.10.2019 16:30

Health, 06.10.2019 16:30


History, 06.10.2019 16:30

Mathematics, 06.10.2019 16:30

Computers and Technology, 06.10.2019 16:30


History, 06.10.2019 16:30



Mathematics, 06.10.2019 16:30

Computers and Technology, 06.10.2019 16:30

Mathematics, 06.10.2019 16:30

Mathematics, 06.10.2019 16:30

Mathematics, 06.10.2019 16:30