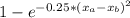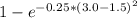Chemistry, 28.01.2020 20:48 Tabbicat021
The compound aluminum nitride () is a compound semiconductor having mixed ionic and covalent bonding. the electronegativities for and are 1.5 and 3.0 respectively. calculate the fraction of the bonding that is ionic. (enter your answer to three significant figures.) =

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
The compound aluminum nitride () is a compound semiconductor having mixed ionic and covalent bonding...
Questions


Mathematics, 11.09.2019 02:30

English, 11.09.2019 02:30


English, 11.09.2019 02:30



Mathematics, 11.09.2019 02:30


English, 11.09.2019 02:30




Computers and Technology, 11.09.2019 02:30




English, 11.09.2019 02:30