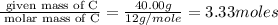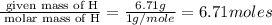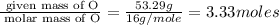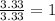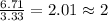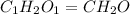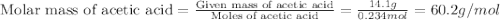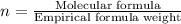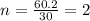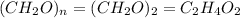Chemistry, 28.01.2020 19:48 kaylallangari549
Acetic acid is an organic compound composed of 40.00% c, 6.71% h, and the rest oxygen. if 0.234 mol of acetic acid has a mass of 14.1 g, what are the empirical and molecular formulas of acetic acid?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 23.06.2019 09:00
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2
You know the right answer?
Acetic acid is an organic compound composed of 40.00% c, 6.71% h, and the rest oxygen. if 0.234 mol...
Questions

Social Studies, 27.10.2020 22:20


Biology, 27.10.2020 22:20

English, 27.10.2020 22:20

World Languages, 27.10.2020 22:20


Social Studies, 27.10.2020 22:20

Chemistry, 27.10.2020 22:20

History, 27.10.2020 22:20

Mathematics, 27.10.2020 22:20

Arts, 27.10.2020 22:20

Health, 27.10.2020 22:20

Mathematics, 27.10.2020 22:20

English, 27.10.2020 22:20






History, 27.10.2020 22:20

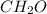 and
and 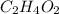 respectively.
respectively.