Chemistry, 28.01.2020 11:31 rodriguezangelinaado
Need 3
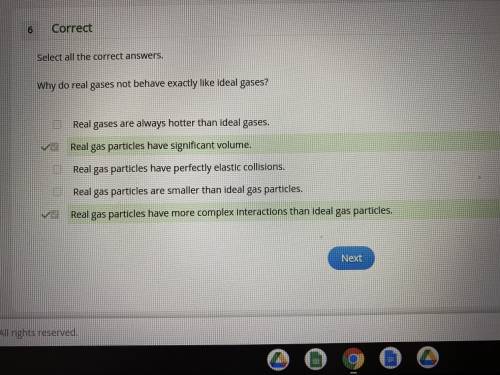
why do real gases not behave exactly like ideal gases?
real gases are always hotter than ideal gases.
real gas particles have significant volume.
real gas particles have perfectly elastic collisions.
real gas particles are smaller than ideal gas particles.
real gas particles have more complex interactions than ideal gas particles.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
Need 3
why do real gases not behave exactly like ideal gases?
real gases are alw...
why do real gases not behave exactly like ideal gases?
real gases are alw...
Questions

Mathematics, 20.01.2021 02:50

Law, 20.01.2021 02:50



Mathematics, 20.01.2021 02:50

Mathematics, 20.01.2021 02:50

Mathematics, 20.01.2021 02:50


Mathematics, 20.01.2021 02:50






Mathematics, 20.01.2021 02:50


History, 20.01.2021 02:50


History, 20.01.2021 02:50

Mathematics, 20.01.2021 02:50