Asample of 0.900 mol n2o is placed in a sealed
container, where it decomposes irreversibly to...

Chemistry, 28.01.2020 08:31 jessicajose4238
Asample of 0.900 mol n2o is placed in a sealed
container, where it decomposes irreversibly to n2 and o2
in a first-order reaction. after 42.0 min, 0.640 mol n2o
remains. how long will it take for the reaction to be
90.0% complete?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1
You know the right answer?
Questions


Mathematics, 02.02.2021 03:10

Mathematics, 02.02.2021 03:10



Mathematics, 02.02.2021 03:10

Chemistry, 02.02.2021 03:10

Social Studies, 02.02.2021 03:10





Mathematics, 02.02.2021 03:10



Mathematics, 02.02.2021 03:10


Chemistry, 02.02.2021 03:10

History, 02.02.2021 03:10

Mathematics, 02.02.2021 03:10

![r=-\frac{d[N_2O]}{dt}= k[N_2O]\\ \\ \frac{d[N_2O]}{dt}= -k[N_2O]](/tpl/images/0475/9615/4d9bd.png)
![\frac{d[N_2O]}{[N_2O]}=-kdt\\ \\ ln[N_2O]-ln[N_2O]_0=-kt](/tpl/images/0475/9615/af464.png)
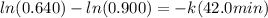 k = 0.00812 min⁻¹
k = 0.00812 min⁻¹

