
Chemistry, 28.01.2020 06:31 daebreonnakelly
Many double-displacement reactions are enzyme-catalyzed via the "ping pong" mechanism, so called because the reactants appear to bounce off the enzyme like a ping pong ball. these reactions typically have two reactants and two products. in a third-order reaction involving two reactants and two products, doubling the concentration of the first reactant causes the rate to increase by a factor of 2. what will happen to the rate of this reaction if the concentration of the second reactant is cut in half?
a. it will increase by a factor of 2.b. it will increase by a factor of 4.c. it will decrease by a factor of 2.d. it will decrease by a factor of 4.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
Many double-displacement reactions are enzyme-catalyzed via the "ping pong" mechanism, so called bec...
Questions


History, 08.04.2020 07:40

English, 08.04.2020 07:40


Mathematics, 08.04.2020 07:40


Mathematics, 08.04.2020 07:40



Mathematics, 08.04.2020 07:41

Mathematics, 08.04.2020 07:41



Advanced Placement (AP), 08.04.2020 07:41

Mathematics, 08.04.2020 07:41



Mathematics, 08.04.2020 07:41

Mathematics, 08.04.2020 07:41

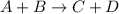
![Rate\ \alpha [A]^{a}[B]^{b}](/tpl/images/0475/5480/f8842.png)
![r=[A]^{a}[B]^{b}](/tpl/images/0475/5480/c58c7.png) .................(1)
.................(1)![r'=[A']^{a}[B']^{b}](/tpl/images/0475/5480/0011b.png)
![2r=[2A]^{a}[B]^{b}](/tpl/images/0475/5480/2ac75.png) ...........(2)
...........(2)![\frac{2r}{r}=\frac{[2A]^{2}[B]^{b}}{[A]^{a}[B]^{b}}](/tpl/images/0475/5480/d883c.png)
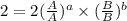
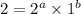
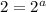
![r=[A]^{1}[B]^{2}](/tpl/images/0475/5480/1a838.png) .............(3)
.............(3)![r'=[A]^{1}[B']^{2}](/tpl/images/0475/5480/b76a3.png)
![r'=[A]^{1}(\frac{1}{2}[B])^{2}](/tpl/images/0475/5480/b0792.png)
![r'=\frac{1}{4}[A]^{1}[B]^{2}](/tpl/images/0475/5480/6503f.png) ............(a)
............(a)

