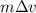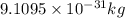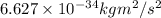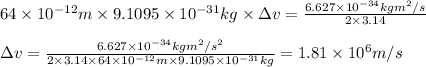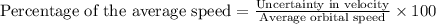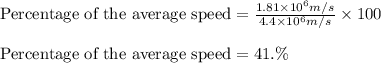An atom of helium has a radius of 31. pm and the average orbital speed of the electrons in it is about 4.4x 10 m/s calculate the least possible uncertainty in a measurement of the speed of an electron in an atom of helium. write your answer as a percentage of the average speed, and round it to 2 significant digits x 10

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
An atom of helium has a radius of 31. pm and the average orbital speed of the electrons in it is abo...
Questions

Computers and Technology, 06.09.2020 03:01





Chemistry, 06.09.2020 03:01



Chemistry, 06.09.2020 03:01



Mathematics, 06.09.2020 03:01

Chemistry, 06.09.2020 03:01

English, 06.09.2020 03:01

Geography, 06.09.2020 03:01


Physics, 06.09.2020 03:01

History, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01

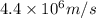 . Calculate the least possible uncertainty in a measurement of the speed of an electron in an atom of helium. Write your answer as a percentage of the average speed, and round it to 2 significant digits
. Calculate the least possible uncertainty in a measurement of the speed of an electron in an atom of helium. Write your answer as a percentage of the average speed, and round it to 2 significant digits (Conversion factor:
(Conversion factor: 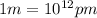 )
)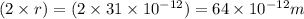
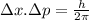
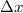 = uncertainty in position = d =
= uncertainty in position = d = 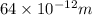
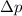 = uncertainty in momentum =
= uncertainty in momentum =