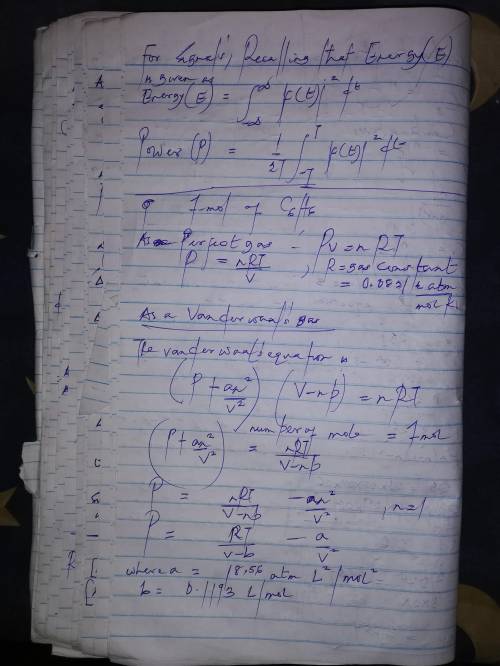Chemistry, 28.01.2020 02:31 Desinfektionsmittel
Calculate the pressure exerted by 1.0 mol of c6h6behaving as a)a perfect gas and b) a van der waals gas when it is confined under the following conditions: i)373.15 k in 22.414 dm3ii)1000 k in 22.414 dm3iii)1000 k in 150.000 dm3at which one of these conditions does the real gas be?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1


Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Calculate the pressure exerted by 1.0 mol of c6h6behaving as a)a perfect gas and b) a van der waals...
Questions

English, 25.09.2019 12:20

History, 25.09.2019 12:20








Biology, 25.09.2019 12:20

Mathematics, 25.09.2019 12:20

Spanish, 25.09.2019 12:20

Mathematics, 25.09.2019 12:20

Social Studies, 25.09.2019 12:20

Mathematics, 25.09.2019 12:20

History, 25.09.2019 12:20