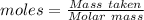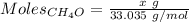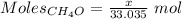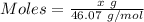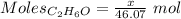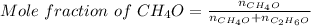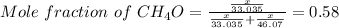Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1


Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
Asolution is made by mixing equal masses of methanol, ch 4 o , and ethanol, c 2 h 6 o . determine th...
Questions



Mathematics, 19.09.2019 17:10


Mathematics, 19.09.2019 17:10

Health, 19.09.2019 17:10

Physics, 19.09.2019 17:10

Mathematics, 19.09.2019 17:10




Health, 19.09.2019 17:10

Mathematics, 19.09.2019 17:10





Computers and Technology, 19.09.2019 17:10


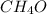 = 0.58
= 0.58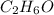 = 0.42
= 0.42