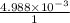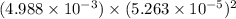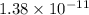Chemistry, 28.01.2020 01:31 SchoolSucks234
The solubility product of calcium fluoride (caf2(s); fluorite) is 310-11 at 25c. could a fluoride concentration of 1.0 mg l-1 be obtained in water that contains 200 mg l-1 of calcium?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
The solubility product of calcium fluoride (caf2(s); fluorite) is 310-11 at 25c. could a fluoride...
Questions





Advanced Placement (AP), 13.10.2020 14:01


Mathematics, 13.10.2020 14:01


Mathematics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01


Mathematics, 13.10.2020 14:01



Mathematics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01


History, 13.10.2020 14:01

English, 13.10.2020 14:01

History, 13.10.2020 14:01

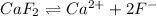
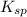 will be as follows.
will be as follows.![K_{sp} = [Ca^{2+}][F^{-}]^{2}](/tpl/images/0474/4010/1d9c8.png)
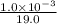
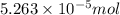
![[F^{-}] = \frac{\text{moles of F^{-}}{volume}](/tpl/images/0474/4010/e0719.png)
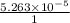
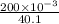
![[Ca^{2+}] = \frac{moles of Ca^{2+}}{volume}](/tpl/images/0474/4010/ce6ee.png)