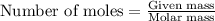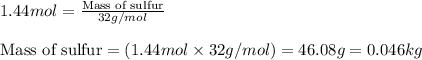Chemistry, 28.01.2020 00:31 lolfunny124
Sulfuric acid is essential to dozens of important industries from steelmaking to plastics and pharmaceuticals. more sulfuric acid is made than any other industrial chemical, and world production exceeds per year. the first step in the synthesis of sulfuric acid is usually burning solid sulfur to make sulfur dioxide gas. suppose an engineer studying this reaction introduces of solid sulfur and of oxygen gas at into an evacuated tank. the engineer believes for the reaction at this temperature. calculate the mass of solid sulfur he expects to be consumed when the reaction reaches equilibrium. round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
Sulfuric acid is essential to dozens of important industries from steelmaking to plastics and pharma...
Questions




Computers and Technology, 26.06.2019 18:20








Geography, 26.06.2019 18:20

Physics, 26.06.2019 18:20


Geography, 26.06.2019 18:20

Geography, 26.06.2019 18:20

Geography, 26.06.2019 18:20

Geography, 26.06.2019 18:20

History, 26.06.2019 18:20

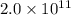 per year.
per year. 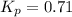 for the reaction at this temperature.
for the reaction at this temperature. 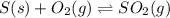
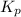 for above equation follows:
for above equation follows: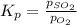
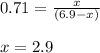
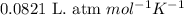
![950^oC=[950+273]K=1223K](/tpl/images/0474/1207/8397a.png)
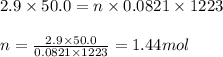
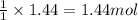 of sulfur
of sulfur