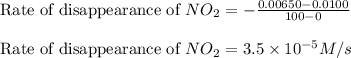Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2no2 → 2no + o2 in a parti...
Questions

Mathematics, 26.03.2020 00:30



Mathematics, 26.03.2020 00:30



History, 26.03.2020 00:30

History, 26.03.2020 00:30

Mathematics, 26.03.2020 00:30








Mathematics, 26.03.2020 00:31

History, 26.03.2020 00:31


History, 26.03.2020 00:31

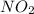 is
is 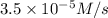
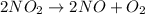
![\text{Rate of disappearance of }NO_2=-\frac{\Delta [NO_2]}{\Delta t}](/tpl/images/0473/6156/ea698.png)

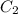 = final concentration of
= final concentration of 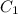 = initial concentration of
= initial concentration of  = final time = 100 minutes
= final time = 100 minutes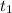 = initial time = 0 minutes
= initial time = 0 minutes