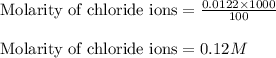Suppose of zinc chloride is dissolved in of a aqueous solution of ammonium sulfate. calculate the final molarity of zinc cation in the solution. you can assume the volume of the solution doesn't change when the zinc chloride is dissolved in it. round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Suppose of zinc chloride is dissolved in of a aqueous solution of ammonium sulfate. calculate the fi...
Questions

Physics, 27.01.2021 03:40


Mathematics, 27.01.2021 03:40


Mathematics, 27.01.2021 03:40

Biology, 27.01.2021 03:40



Mathematics, 27.01.2021 03:40

English, 27.01.2021 03:40


Mathematics, 27.01.2021 03:40



Mathematics, 27.01.2021 03:40

Mathematics, 27.01.2021 03:40

Mathematics, 27.01.2021 03:40

Biology, 27.01.2021 03:40


Mathematics, 27.01.2021 03:40

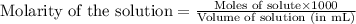 .....(1)
.....(1)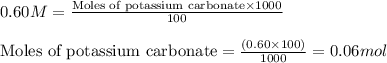
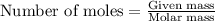
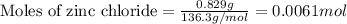
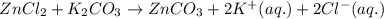
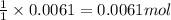 of potassium carbonate
of potassium carbonate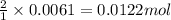 of acetate ion
of acetate ion