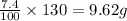Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
Asolid mixture consists of 47.6g of kno3 (potassium nitrate) and 8.4g of k2so4 (potassium sulfate)....
Questions



History, 05.02.2021 22:40

History, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40



Arts, 05.02.2021 22:40

Social Studies, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40

Business, 05.02.2021 22:40

History, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40