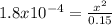Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1


Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
Calculate the ph for the following weak acid.
a solution of hcooh has 0.15m hcooh at equ...
a solution of hcooh has 0.15m hcooh at equ...
Questions

Mathematics, 04.11.2021 19:50

Biology, 04.11.2021 19:50


Business, 04.11.2021 19:50



Mathematics, 04.11.2021 19:50



English, 04.11.2021 19:50

Chemistry, 04.11.2021 19:50

History, 04.11.2021 19:50

Mathematics, 04.11.2021 19:50

Mathematics, 04.11.2021 19:50





Mathematics, 04.11.2021 19:50

![ka = \frac{[H3O][HCOO]}{[HCOOH]}](/tpl/images/0471/8959/57b44.png)
![1.8 x 10^{-4} = \frac{[x][x]}{0.15 - x}](/tpl/images/0471/8959/c09c0.png)