
Chemistry, 27.01.2020 01:31 eagles2286
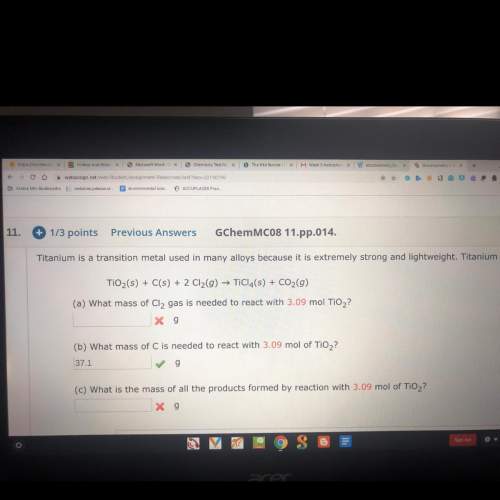
Titanium is a transition metal used in many alloys because it is extremely strong and lightweight. titanium tetrachloride (ticl4) is extracted from titanium oxide using chloride and coke (carbon).
a) what mass of cl2 gas is needed to react with 3.09 mol tio2?
c) what is the mass of all the products formed by reaction with 3.09 mol tio2

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Titanium is a transition metal used in many alloys because it is extremely strong and lightweight. t...
Questions

Chemistry, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01


English, 23.10.2020 01:01

Social Studies, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01

Social Studies, 23.10.2020 01:01

English, 23.10.2020 01:01

Health, 23.10.2020 01:01

History, 23.10.2020 01:01






Advanced Placement (AP), 23.10.2020 01:01



English, 23.10.2020 01:01



