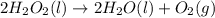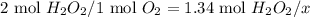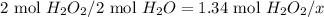Dihydrogen dioxide decomposes into water and oxygen gas. calculate the amounts requested if 1.34 moles of dihydrogen dioxide react according to the equation.
you must show all units.
a. moles of oxygen formed
b. moles of water formed
c. mass of water formed
d. mass of oxygen formed

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
Dihydrogen dioxide decomposes into water and oxygen gas. calculate the amounts requested if 1.34 mol...
Questions

Mathematics, 26.12.2019 04:31





History, 26.12.2019 04:31

Mathematics, 26.12.2019 04:31

Mathematics, 26.12.2019 04:31

Chemistry, 26.12.2019 04:31




History, 26.12.2019 04:31



Mathematics, 26.12.2019 04:31


Chemistry, 26.12.2019 04:31

Mathematics, 26.12.2019 04:31