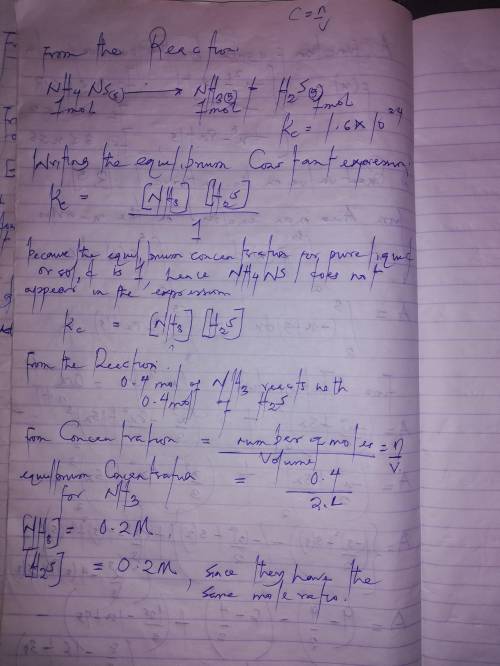Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

You know the right answer?
When solid nh₄hs and 0.400 mol nh₃(g) were placed in a vessel of volume 2.0 l at 24°c, the equilibri...
Questions

Mathematics, 26.07.2019 00:00



History, 26.07.2019 00:00

English, 26.07.2019 00:00

Mathematics, 26.07.2019 00:00

Social Studies, 26.07.2019 00:00


Mathematics, 26.07.2019 00:00







Physics, 26.07.2019 00:00

Mathematics, 26.07.2019 00:00



Social Studies, 26.07.2019 00:00