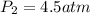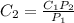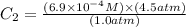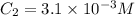Chemistry, 25.01.2020 04:31 James300102
The solubility of nitrogen gas in water at 25 °c and a nitrogen pressure of 1.0 atm is 6.9 × 10-4 m. the solubility of nitrogen in water at a nitrogen pressure of 4.5 atm is m.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
The solubility of nitrogen gas in water at 25 °c and a nitrogen pressure of 1.0 atm is 6.9 × 10-4 m....
Questions

Chemistry, 09.06.2020 20:57


Physics, 09.06.2020 20:57

Physics, 09.06.2020 20:57




Health, 09.06.2020 20:57


History, 09.06.2020 20:57


Chemistry, 09.06.2020 20:57



Mathematics, 09.06.2020 20:57

Mathematics, 09.06.2020 20:57

Mathematics, 09.06.2020 20:57


English, 09.06.2020 20:57

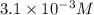
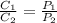
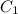 and
and 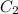 are solubility of the gas at a pressure of
are solubility of the gas at a pressure of 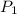 and
and 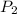 respectively.
respectively.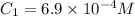 ,
, 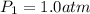 and
and