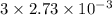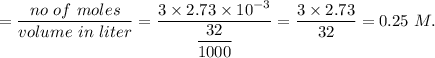Chemistry, 25.01.2020 02:31 Ayyyyeeeeeeewuzgud
A32.00 ml sample of an unknown h3po4 solution is titrated with a 0.110 m naoh solution. the equivalence point is reached when 24.83 ml of naoh solution is added. what is the concentration of the unknown h3po4 solution? the neutralization reaction is
h3po4(aq)+3naoh(aq)→3h2o(l)+na3po4( aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
A32.00 ml sample of an unknown h3po4 solution is titrated with a 0.110 m naoh solution. the equivale...
Questions

Social Studies, 15.12.2019 16:31

History, 15.12.2019 16:31

Social Studies, 15.12.2019 16:31





Computers and Technology, 15.12.2019 16:31





Mathematics, 15.12.2019 16:31


Health, 15.12.2019 16:31

Social Studies, 15.12.2019 16:31




Mathematics, 15.12.2019 16:31

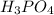 in sample is 0.25 M.
in sample is 0.25 M.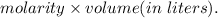
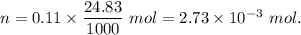
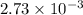 mol of NaOH reacts with
mol of NaOH reacts with