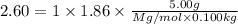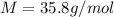Chemistry, 24.01.2020 22:31 freshysans4
The freezing point of an aqueous solution containing an unknown solute is -2.60 degc. the solution was prepared by dissolving 5.00 g of a nonelectrolytic solute in 100. ml of water. what is the molar mass of the unknown solute?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
The freezing point of an aqueous solution containing an unknown solute is -2.60 degc. the solution w...
Questions

History, 12.05.2021 20:20



Business, 12.05.2021 20:20


Physics, 12.05.2021 20:20


Mathematics, 12.05.2021 20:20



Mathematics, 12.05.2021 20:20



Mathematics, 12.05.2021 20:20

Mathematics, 12.05.2021 20:20


Mathematics, 12.05.2021 20:20


Mathematics, 12.05.2021 20:20

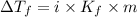
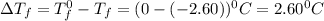 = Depression in freezing point
= Depression in freezing point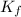 = freezing point constant =
= freezing point constant = 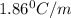
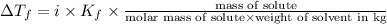
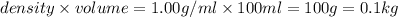 (1kg=1000g)
(1kg=1000g)