
Chemistry, 24.01.2020 21:31 vistagallosky
6. barium sulfate and and sodium sulfate react in a double displacement reaction. if the
reaction starts with 10.25 grams of barium sulfate what are the products and how many moles
of each product is produced?
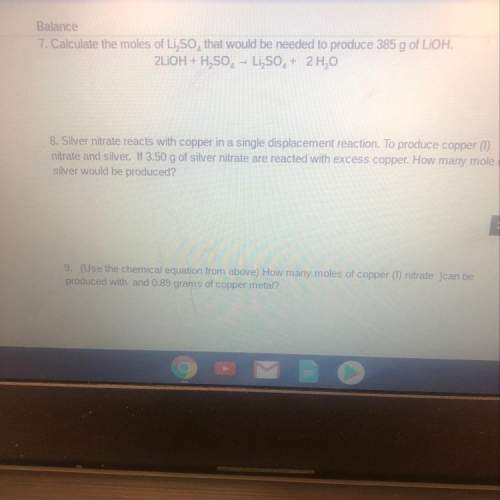
7. calculate the moles of li2so4that would be needed to produce 385 g of lioh.
8. silver nitrate reacts with copper in a single displacement reaction. to produce copper (1)
nitrate and silver. if 3.50 g of silver nitrate are reacted with excess copper. how many mole of
silver would be produced?
9. (use the chemical equation from above) how many moles of copper (1) nitrate ]can be
produced with and 0.89 grams of copper metal?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
6. barium sulfate and and sodium sulfate react in a double displacement reaction. if the
react...
react...
Questions

Mathematics, 08.07.2019 16:30

Mathematics, 08.07.2019 16:30

History, 08.07.2019 16:30

Mathematics, 08.07.2019 16:30


Mathematics, 08.07.2019 16:30


Mathematics, 08.07.2019 16:30


English, 08.07.2019 16:30

Mathematics, 08.07.2019 16:30

French, 08.07.2019 16:30

Mathematics, 08.07.2019 16:30

History, 08.07.2019 16:30



Biology, 08.07.2019 16:30


Mathematics, 08.07.2019 16:30



